Abstract
Background: FVIII deficient knock-out (F8 -/-) rats mimic the bleeding incidents seen in severe human hemophilia A (HA). Subcutaneous (SQ) marzeptacog alfa activated (MarzAA), a novel, engineered recombinant activated coagulation Factor VII (rFVIIa) has been shown to effectively treat episodic bleeding in a pilot experiment in HA rats. This study evaluated the effect of single SQ doses of MarzAA and a single intravenous (IV) dose of rFVIIa on episodic bleeding in F8 -/- rats. Moreover, it compared the effect of SQ MarzAA and IV rFVIIa directly.
Methods: Animals were allocated to treatment with either SQ vehicle, SQ MarzAA (60, 180 or 385 µg/kg) or IV rFVIIa (NovoSeven ®) (580 µg/kg) immediately after the bleeding was diagnosed. Doses were based on allometric scaling from humans (Nair AB and Jacob S. J Basic Clin Pharm 2016; 7: 27-31). The primary endpoint of the study was clinical efficacy as rated by a well-defined 4-point scale (Excellent, Good, Fair or Poor), and the efficacy assessment was either treatment success (Excellent or Good) or treatment failure (Fair or Poor) at the 24-hour timepoint. All personnel handling or assessing animals were blinded to the treatment status of each animal except those dosing animals who knew the route of administration.
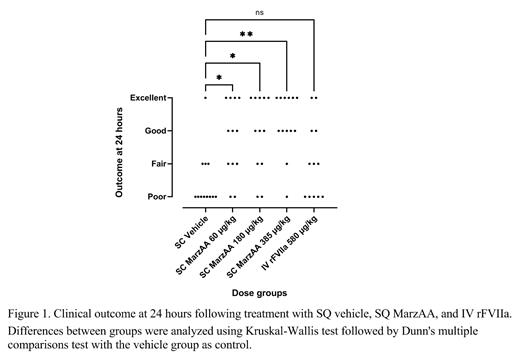
Results: A total of 86 F8 -/- rats was enrolled in the study. Of these, 61 rats presented treatment eligible bleeds between 3 and 10 weeks of age. No statistically significant difference in bleeding severity score were found across groups on diagnosis. As assessed by the clinical outcome at 24 hours, all three SQ MarzAA dose groups exhibited a statistically significant effect on treatment response when compared to SQ vehicle treatment (Figure 1). Conversely, no statistically significant effect could be identified when the single IV rFVIIa 580 µg/kg dose group was compared to vehicle. The overall treatment success rates at 24 hours were: SQ vehicle: 8%, SQ MarzAA 60 µg/kg: 58%, SQ MarzAA 180 µg/kg: 67%, SQ MarzAA 385 µg/kg: 85%, and IV rFVIIa 580 µg/kg: 33%. When compared directly using allometric scaling of clinical doses, SQ MarzAA at 385 µg/kg exhibited a statistically superior effect compared to IV rFVIIa at 580 µg/kg (p=0.0154, Fischer's exact test).
Conclusion: Single doses of SQ MarzAA were effective in treating episodic bleeding in HA rats and statistically distinguishable from vehicle at all dose levels tested. When clinically relevant doses were compared directly to rFVIIa, SQ MarzAA compared favorably to IV rFVIIa. Taken together, these data provide robust nonclinical evidence that a single dose of SQ MarzAA may be successful in treating episodic bleeding when administered after bleeding has started.
Knudsen: Catalyst Biosciences: Current Employment, Current holder of individual stocks in a privately-held company. Blouse: Catalyst Biosciences: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal